Learn How Lithium-ion Batteries Work
Lithium-ion batteries, or lithium-ion batteries for short, are a type of rechargeable battery that has become increasingly popular in recent years. The basic design of a lithium-ion battery consists of an anode (positive electrode), a cathode (negative electrode), an electrolyte and a separator.
1. Electrodes: The positively and negatively charged ends of a cell. Attached to the current collectors.
2. Cathode: The positive electrode.
3. Anode: The negative electrode.
4. Electrolyte: A liquid or gel that conducts electricity.
5. Separator: A porous polymeric film that separates the electrodes while enabling the exchange of lithium ions from one side to the other.
6. Current collectors: Conductive foils at each electrode of the battery that are connected to the terminals of the cell. The cell terminals transmit the electric current between the battery, the device and the energy source that powers the battery.
Lithium-ion Batteries working principle is shown in the figure:
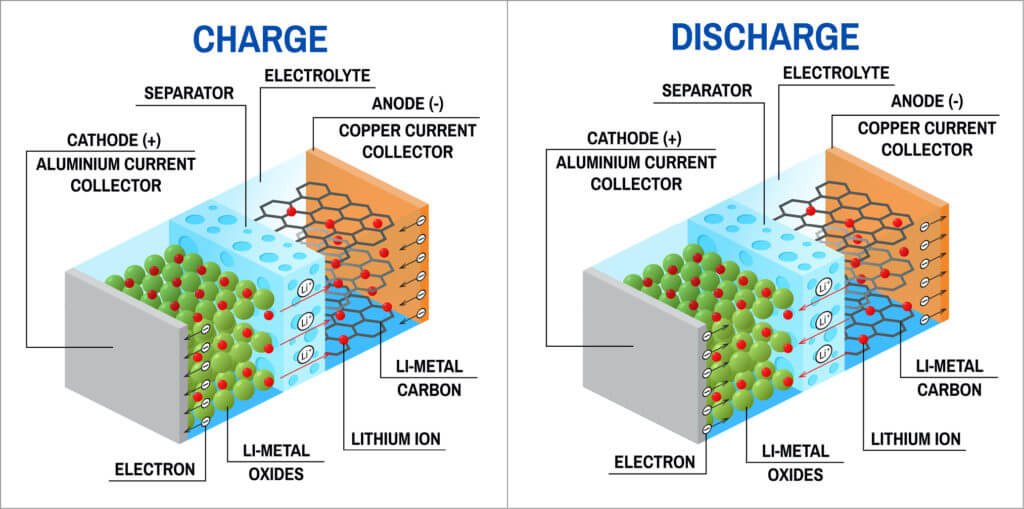
Bright Power How Lithium-ion Batteries Work
The anode in a lithium-ion battery is typically made of graphite, which can store lithium ions. The cathode is typically made of a metal oxide, such as lithium cobalt oxide or lithium iron phosphate, which can also store lithium ions. When the battery is being charged, lithium ions move from the cathode to the anode through the electrolyte, while electrons flow through an external circuit and are collected by the positive current collector.
During discharge, the process is reversed. Lithium ions move from the anode to the cathode, releasing energy that can be used to power devices. Electrons flow through the external circuit from the negative current collector to the positive current collector. The separator, which is typically made of a porous material, prevents the anode and cathode from coming into contact with each other and causing a short circuit.
The electrolyte in a lithium-ion battery is typically a lithium salt dissolved in an organic solvent, such as ethylene carbonate or diethyl carbonate. The electrolyte allows the flow of lithium ions between the anode and cathode during charging and discharging cycles.
In summary, the working of a lithium-ion battery involves the movement of lithium ions between the anode and cathode during charging and discharging cycles, facilitated by the electrolyte. This movement of ions creates a flow of electrons through an external circuit that can be used to power devices.